Adolescence is an exciting time! Alongside puberty, adolescents undergo marked development in cognitive, social, and emotional skills. But it is also a time of increased vulnerability to mental health disorders: 50% of adolescents meet the criteria for a mental health disorder; in 22% the impairment is severe1. Many of these disorders are more common or more severe in one sex than the other. What is responsible for these adolescent behavioral changes? Why is this a time of increased vulnerability to mental health disorders? And what is responsible for the differences between adolescent boys and girls?
Our lab studies potential neural and hormonal contributions to behavioral changes and mental health disorders that arise during adolescence, with a particular focus on why there are differences between males and females. To do this, we use animal models that are ideally suited for these questions. We use a variety of behavioral tests, but our most common measures are social play and ultrasonic vocalizations. Below is a description of these behavioral measures followed by descriptions of two active projects in the lab.
Social Play
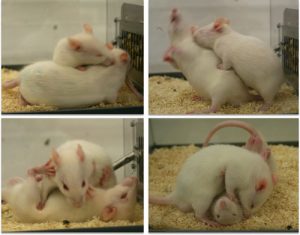
For most species, social play is rough-and-tumble play or wrestling. This behavior is seen in juveniles and adolescents of most mammalian species from rats to dogs, humans, lions, tigers, and bears, oh my! Play is ideal for developmental studies because: 1) it is important for social, emotional, and cognitive development; 2) it has a well-characterized developmental profile which peaks in early adolescence then declines in adulthood; and 3) children with developmental disorders often show deficits in play.
Ultrasonic Vocalizations
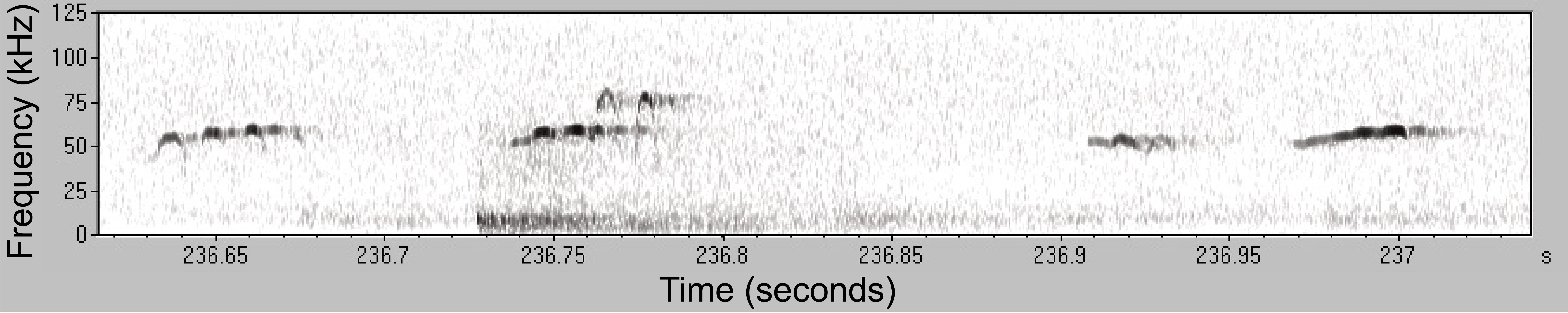
Ultrasonic vocalizations (USVs) are very high frequency sounds made by rats (and some other species) that are out of our hearing range. Nevertheless, the rats can hear them, and this is one way they communicate with each other. USVs with frequencies around 50 kHz are thought to represent positive affect (rats are happy!); those with frequencies around 22kHz are thought to represent fear or distress. When we study rats playing, virtually all USVs are in the 50kHz range – yay! Rats will often modulate the frequency of their call leading to cool patterns on the spectrogram.
Project 1. The role of vasopressin in social development: Investigations into the socially awkward Brattleboro rat.
Vasopressin is a protein made in the brain (a.k.a. neuropeptide) that regulates a diverse set of physiological functions including behavioral timing, stress, autonomic function, and water balance. In addition to these physiological actions, vasopressin has long been known to influence many social behaviors in adult animals and humans. However, its role in social development has only recently been assessed. Furthermore, it is still not clear how vasopressin influences social behavior in adults or during development. To address these questions, we are using a rat that has a natural mutation in the vasopressin gene – the Brattleboro rat.

The Brattleboro mutation was discovered in a laboratory near Brattleboro, VT (hence the name). This mutation disrupts the production of vasopressin, and rats with two copies of the Brattleboro mutation cannot produce functional vasopressin (we each get two copies of a gene, one from mom and one from dad). Remember when we said that vasopressin influences water balance? Well, it does so by helping the kidneys retain water from urea. Brattleboro rats cannot do this, so they lose most of their water in their urine, which causes them to urinate and drink excessively – a condition called diabetes insipidus. This is actually how they were discovered because their cages were always soiled and their water bottles needed to be changed every day or so. Don’t worry, as long as they are given enough water and their cages are cleaned frequently, they can live healthy lives.
So do Brattleboro rats have deficits in their social behaviors during development? When we asked this question, we found something surprising – Brattleboro rats show fewer active social behaviors (e.g., play and prosocial USVs), but more passive social behaviors (e.g., huddling). So Brattleboro rats are not asocial, but rather their social behavior is atypical (see Paul et al. 2016 eNeuro ). This has lead us to rethink how vasopressin might be influencing social behavior. We now think that vasopressin influences the type of social behavior expressed through actions on arousal. We are currently testing this hypothesis and asking which vasopressin pathway is responsible for vasopressin’s actions during development.
Project 2. Adolescent behavior: Are pubertal hormones to blame?
Although often used interchangeably, puberty and adolescence are not the same. Puberty refers to the activation of the reproductive axis and the consequent increase in gonadal hormones, appearance of secondary sexual characteristics, and the ability to reproduce. Adolescence includes these pubertal changes, but also includes social, emotional, and cognitive changes that occur during the teenage years and early twenties. So one obvious question is – does the activation of the reproductive axis at puberty causes these other non-reproductive adolescent changes? In other words, is the crazy behavior of teenagers due to raging pubertal hormones? To answer this question, it would be easiest if we could control the timing of puberty and hold an individual in the prepubertal state to see if this delay in puberty affects non-reproductive adolescent changes. You can’t do this in a human; nor would we want to! However, we are working with a model where you can do this – enter Siberian hamsters!

Siberian hamsters have many seasonal adaptations, including changes in fur, body weight, and immune function. One seasonal adaptation in particular makes them ideal for our experiments – Siberian hamsters only breed in the spring and summer when conditions are favorable. It is hard to raise young in the middle of a Siberian winter! Siberian hamsters born in the beginning of the breeding season undergo rapid pubertal development in order to breed that same year, however, those born at the end of the breeding season delay puberty by 3-5 months in order to synchronize the onset of their breeding with the following spring. We can recapitulate this early versus delayed puberty in the lab simply by manipulating the ambient light/dark cycle. Hamsters reared in a long summer-like day length (e.g., 15 h light/day) undergo rapid pubertal development, whereas those reared in a short, winter-like day length (e.g., 10 h light/day) delay puberty. So in this species, we can use the day/night cycle (a.k.a. photoperiod) to control when hamsters begin puberty! We are now using this model to see which behaviors and neural circuits are regulated by pubertal hormones (puberty-dependent traits) and which are not (puberty-independent traits). See the figure below from our Journal of Neuroscience paper for more details on the rationale of this approach.
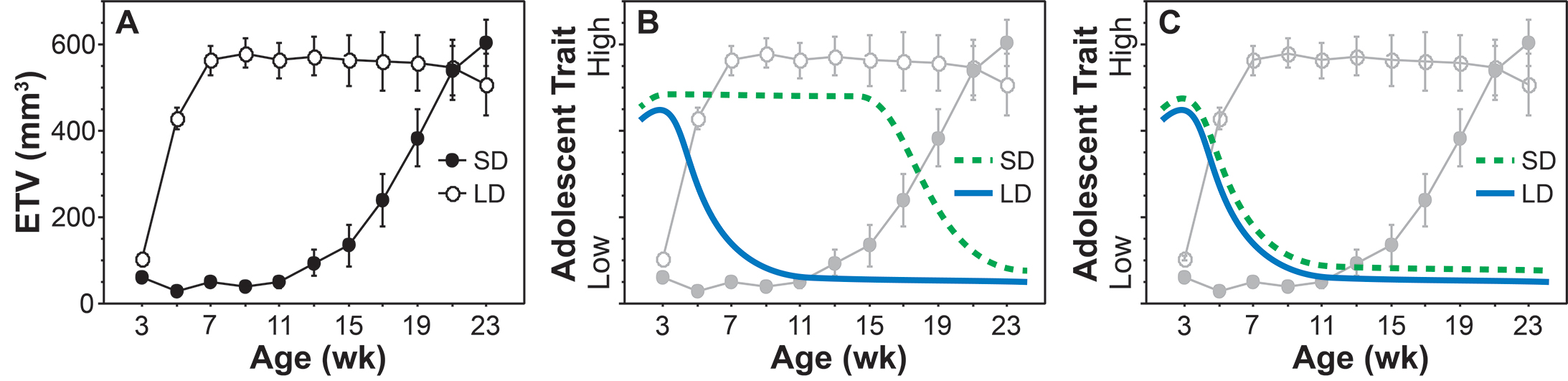
Figure 2 from Walker et al. 2017 J Neurosci 37(45):10855-10866. Using seasonal breeders to separate puberty-dependent and puberty-independent influences on adolescent development. Panel A depicts photoperiod regulation of puberty in male Siberian hamsters: long, summer-like day lengths (LD) stimulate, whereas short, winter-like day lengths (SD) delay, testicular development. Panels B and C illustrate how this model can be used to determine whether the development of an adolescent trait is regulated by puberty-dependent or puberty-independent mechanisms. The developmental profile of a hypothetical adolescent trait is depicted as a solid blue line for LD-reared hamsters and a dashed green line for SD-reared hamsters. Delayed development of the adolescent trait in SD-reared hamsters (as in B) maintains temporal synchrony with puberty and indicates puberty-dependent regulation. Absence of a photoperiod effect on the adolescent trait (as in C), however, dissociates its development from puberty in SD-reared hamsters and indicates puberty-independent regulation. ETV = estimated testis volume. Illustration of testis data modified from figure 2 in Paul et al. 2006 Biol Reprod 75:261-269.
So, which behaviors are puberty-dependent and which are puberty-independent? We are just getting started with this approach and the answer seems to be that it depends on the behavior being studied and even the component of the behavior being measured (levels of behavior versus timing of developmental changes). In our recent publication in Current Biology, we looked at the transition from juvenile social play to adult aggression, which occurs in many rodents during adolescence. We found that pubertal hormones had no effect on the timing of the transition from play to aggression! However, gonadal hormones did impact whether hamsters displayed high levels of play or aggression. Check out the full publication: Paul et al. 2018 Curr Biol 28(7):1116-1123. Our photo for this publication made the cover!

Now we are applying this approach to other behaviors (e.g., exploration and risk taking) and to neural circuits that continue to develop across adolescence (e.g., vasopressin).
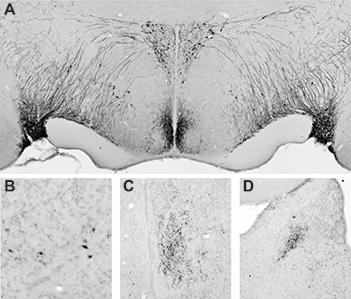
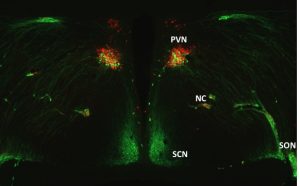
Vasopressin in the Siberian hamster. Immunohistochemical staining of hypothalamic vasopressin pathways (A) and the forebrain vasopressin pathway (B,C,D), including cell bodies in the bed nucleus of the stria terminalis (B) and fiber projections in the lateral septum (C) and lateral habenula (D).
1Merikangas et al. 2010 J Am Chil Adolesc Psychiatry 49(10):980-989
