Coordination-driven self-assembly is a synthetic methodology capable of delivering diverse, tunable architectures, yet is currently underdeveloped as a tool to address the chemistry of energy conversion. In particular, the ability to independently design molecular subunits with specific properties which can combine into multifunctional scaffolds provides a method to generate functionally complex yet synthetically facile materials. The Cook Group exploits coordination-driven self-assembly to explore energy-relevant materials with modular photo-, redox- and catalytically active building blocks that can rapidly incorporate new molecular discoveries relevant to energy harvesting, storage, and use.
Our research program is rooted in fundamental structural, photophysical and electrochemical characterization techniques applied towards discrete supramolecular coordination complexes (SCCs) formed using the principles of molecular self-assembly and coordination chemistry. Primary thrusts include:
(1) Light Harvesting Metallacycles and Cages via Self-Assembly
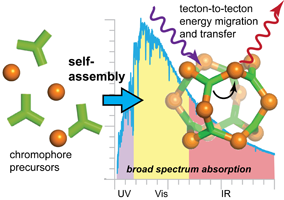
The efficient capture of solar energy requires materials that strongly absorb across the visible spectrum so as to effectively match the solar flux. Chemical approaches to new light harvesting materials often rely on the organization of molecular units, inspired by nature’s photosystems. The precedent for strongly absorbing metal and organic-based molecules motivates our design of discrete supramolecular coordination complexes containing complementary chromophores.
(2) Redox-Active SCCs for Redox Flow Devices
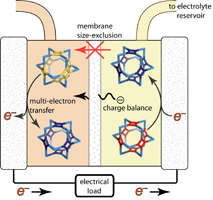 Redox flow batteries (RFBs) break the paradigm of traditional battery designs by separating the charge carriers from the core of the device. The electrolyte chemistries of current RFBs center on small molecules and/or simple ions, which exacerbate electrolyte mixing and decomposition and offer limited tunability. We explore the synthesis and characterization of multinuclear redox-active species in the context of RFB charge carriers to interface with energy capture schemes, thereby developing a rich electrochemistry of inorganic supramolecular complexes.
Redox flow batteries (RFBs) break the paradigm of traditional battery designs by separating the charge carriers from the core of the device. The electrolyte chemistries of current RFBs center on small molecules and/or simple ions, which exacerbate electrolyte mixing and decomposition and offer limited tunability. We explore the synthesis and characterization of multinuclear redox-active species in the context of RFB charge carriers to interface with energy capture schemes, thereby developing a rich electrochemistry of inorganic supramolecular complexes.
(3) Coordination-Driven Self-Assembly of Cofacial Catalysts
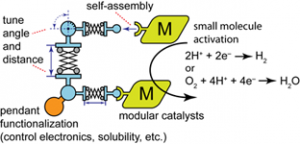
The activation of energy-relevant small molecules is difficult owing to the multi-electron multi-proton nature of this chemistry. Polynuclear designs distribute redox and coordination demands across multiple sites which can act in concert to effect key catalytic steps. The formation of cofacial catalysts can be greatly simplified through the use of coordination-driven self-assembly, wherein two catalytic species can be tethered via distal coordination to pillar building blocks. Since the bridging pillar and catalyst platforms are independently synthesized, the size, shape, flexibility, and chemical properties of resultant cofacial complexes can be readily optimized by orthogonal adjustments, which will not significantly affect self-assembly.
