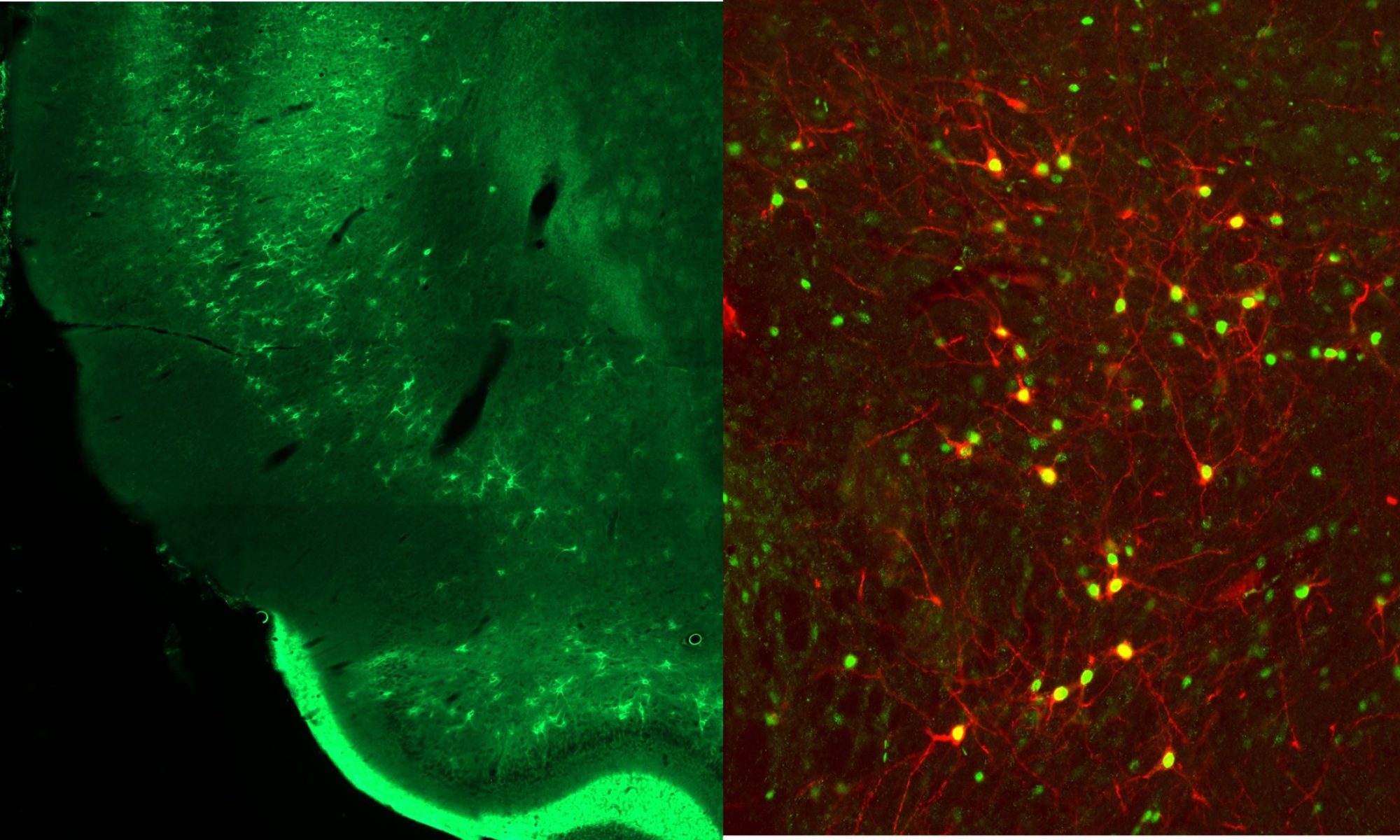
Insular Cortical Mechanisms Contributing to ‘Food Noise’ in Obesity
Greater than 40% of the adult population of the U.S. classify as obese. A major complicating factor in obesity is the persistence of ‘food noise’ in individuals with obesity. In part, food noise is characterized by intense and pervasive intrusive thoughts, cravings, and urges to consume food in response to cues associated with consumption of various foods. As such, food noise can significantly contribute to overconsumption and impair the ability to maintain a healthy eating regimen. Individuals prescribed GLP-1 agonists, such as semaglutide and liraglutide, report a drastic reduction in the prevalence of food noise and cessation of agonist treatment is associated with a profound ‘rebound’ in the presence of food noise and preoccupation with eating. We have been leveraging fiber photometric analyses using genetically encoded biosensors (GCaMP and GRAB-ACh) to demonstrate that food cues are associated with a general increase in neural activity within the insular cortex (GCaMP) and that both these cues and eating drive increases in insular acetylcholine release (GRAB-ACh). These studies are positioned to determine the neural mechanisms of food noise, how GLP-1 agonist treatment works to alleviate the presence of food noise, and the mechanisms via which cessation of GLP-1 agonist treatment results in this rebound in the prevalence of food noise.
The Neurobiology of Nicotine and Opioid Comorbidity
Approximately 85% of individuals currently seeking treatment for an opioid use disorder are habitual users of nicotine-containing products. In preclinical rodent models, nicotine administration substantially augments the self-administration of opioids. Moreover, concurrent nicotine administration engenders a punishment-resistant phenotype in animals responding for intravenous opioids. We have recently demonstrated that nicotine administration directly within the insular cortex is sufficient to interfere with learning about the adverse consequences of acute opioid intoxication. As such, we are currently investigating the role of insular cortical nicotinic signaling on the development of compulsive-like opioid consumption. To this end we employ site-specific behavioral pharmacology and chemogenetic circuit dissection in rodent models.
Neural and Behavioral Parameters Influencing the Escalation of Alcohol Intake
Alcohol use disorder is characterized, in part, by an escalation in alcohol intake and a desire to continue to consume alcohol despite the threat of harmful or adverse repercussions. One adaptation associated with escalation of alcohol intake is an insensitivity, or attentional blindness, to the aversive properties of alcohol. We are particularly interested in elucidating the neural and behavioral adaptations that are driving this decrease in salience. Currently we are exploring the role played by dopaminergic innervation of the insular cortex and how alcohol exposure across the lifespan may alter insular dopaminergic signaling.