The goal of our research program is to develop first-in-class tool compounds and evaluate key aspects of drug development to accelerate new therapies and reduce the burden of human disease.
We take an interdisciplinary approach that integrates medicinal chemistry, structural biology, and pharmacology to tackle complex challenges in early-stage drug discovery. In parallel, we are deeply committed to training the next generation of scientists by providing hands-on experience across these disciplines, fostering a collaborative environment where students learn to think critically, work across fields, and contribute meaningfully to therapeutic innovation.
Covalent Drugs
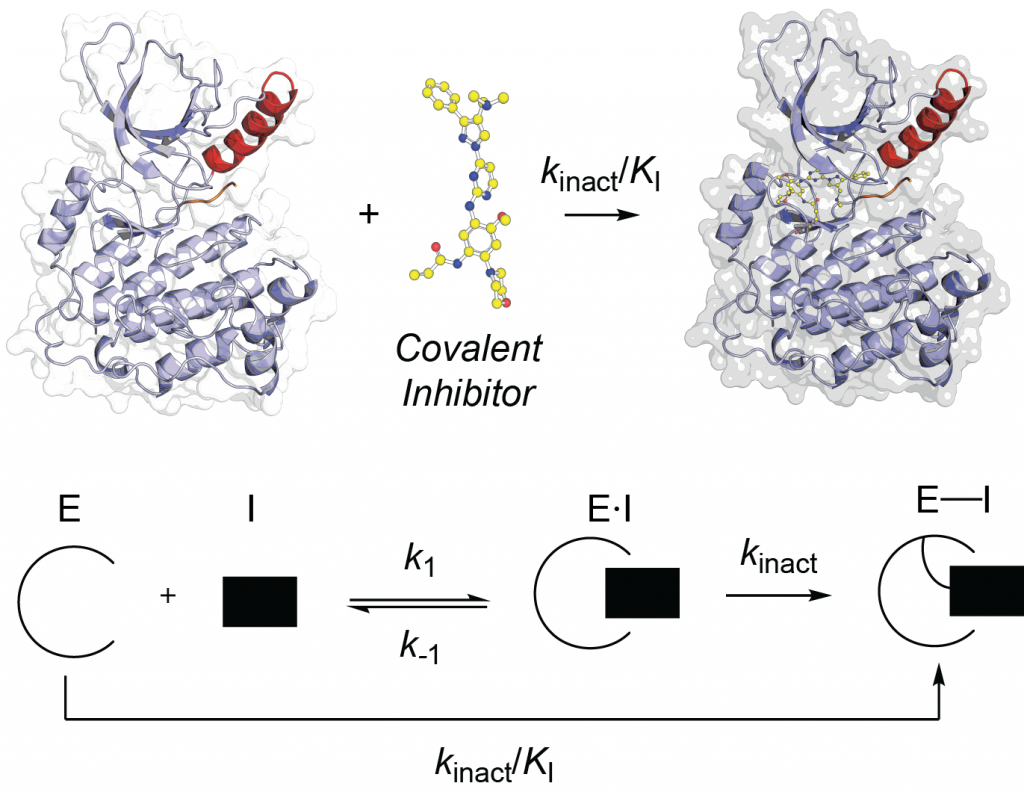
Covalent inhibitors have (re)emerged as powerful tools in drug discovery due to their ability to achieve high potency and prolonged target engagement through irreversible binding. They are particularly valuable for targeting shallow or transient binding pockets and have shown clinical success in areas such as oncology and infectious diseases. We seek to better understand the power of covalent drugs and develop deeper understanding for their characterization and optimization.
- Profiling and Optimizing Targeted Covalent Inhibitors through EGFR-Guided Studies. Journal of Medicinal Chemistry 2025.
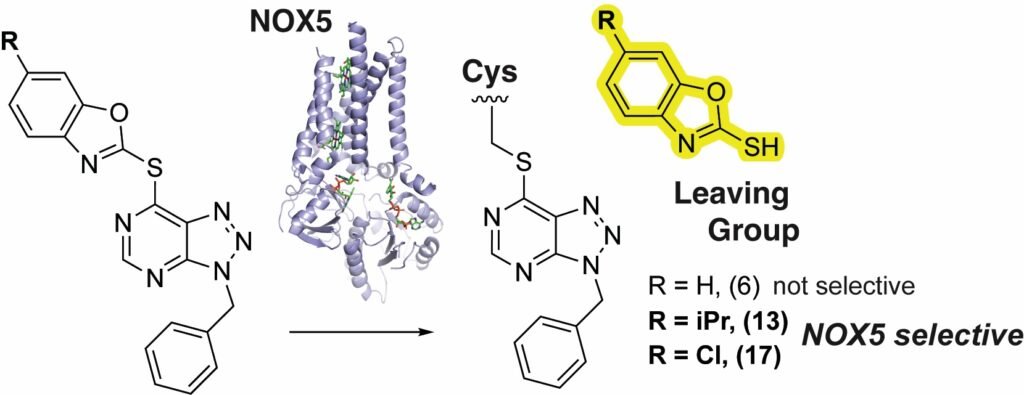
- Enhancing Selectivity and Potency of SNAr Covalent Inhibitors of NADPH Oxidase Enzymes. Journal of Medicinal Chemistry 2025.
- Demystifying Functional Parameters for Irreversible Enzyme Inhibitors. Journal of Medicinal Chemistry 2024.
- Pitfalls and Considerations in Determining the Potency and Mutant Selectivity of Covalent EGFR Inhibitors. Journal of Medicinal Chemistry 2024.
Drug Design Strategies
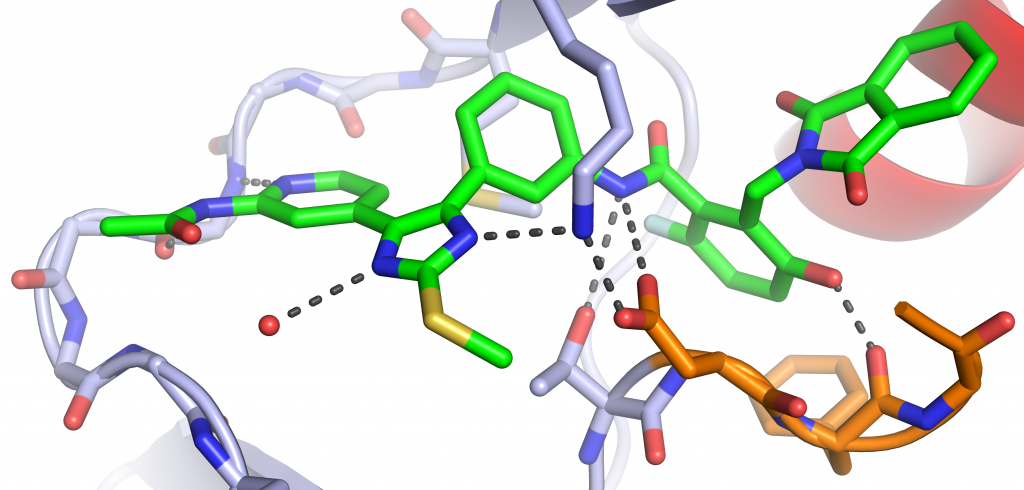
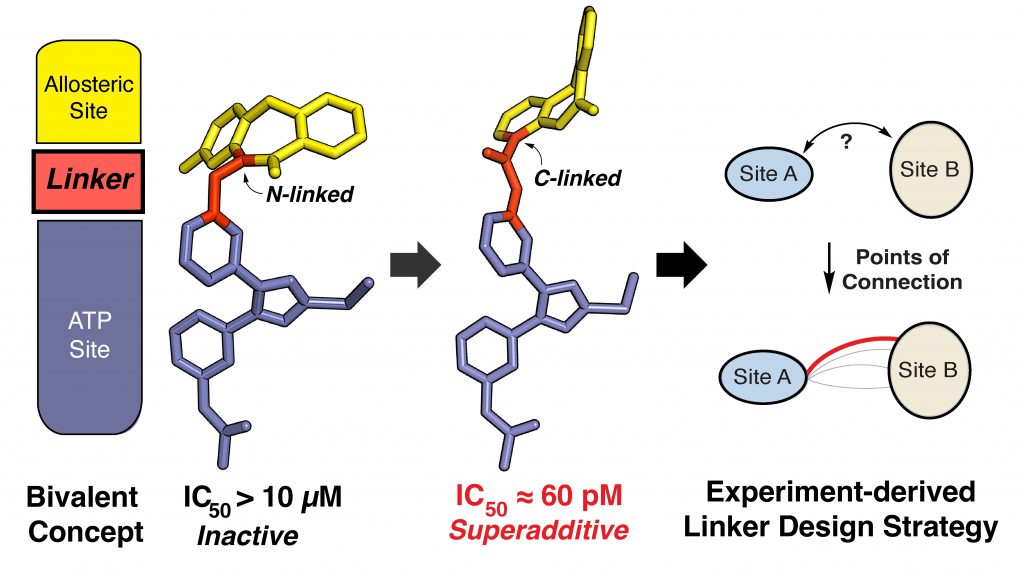
Drug discovery often requires the synthesis of large numbers of compounds, many of which fail to meet biological or pharmacokinetic criteria, making the process time-consuming and resource-intensive. By integrating structural biology and modeling into established targets, we aim to prioritize certain design strategies leading to more efficient and targeted synthesis if highly-active compounds. Our most recent work has concerned the design of ATP-allosteric bivalent inhibitors (AABIs) for better understanding of drug design and targeting activating mutations of the epidermal growth factor receptor (EGFR).
- Structure-activity relationships of inactive-conformation binding EGFR inhibitors: Linking the ATP and allosteric pockets. Archiv der Pharmazie 2025.
- Linking ATP and allosteric sites to achieve superadditive binding with bivalent EGFR kinase inhibitors. Communications Chemistry 2024.
- Design of a “Two-in-One” Mutant-Selective EGFR Inhibitor That Spans the Orthosteric and Allosteric Sites. Journal of Medicinal Chemistry 2022.
Expanding the Scope of Protein Kinase Inhibitors
The continued pursuit of novel kinase inhibitors is essential for addressing drug resistance and expanding treatment options across many human diseases. Advancements in this area have the potential to significantly impact the management of cancers as well as inflammatory disorders like rheumatoid arthritis and neurodegenerative diseases including Parkinson’s disease. On going projects involve exploring new kinase targets taking advantage of structural and biochemical tools.
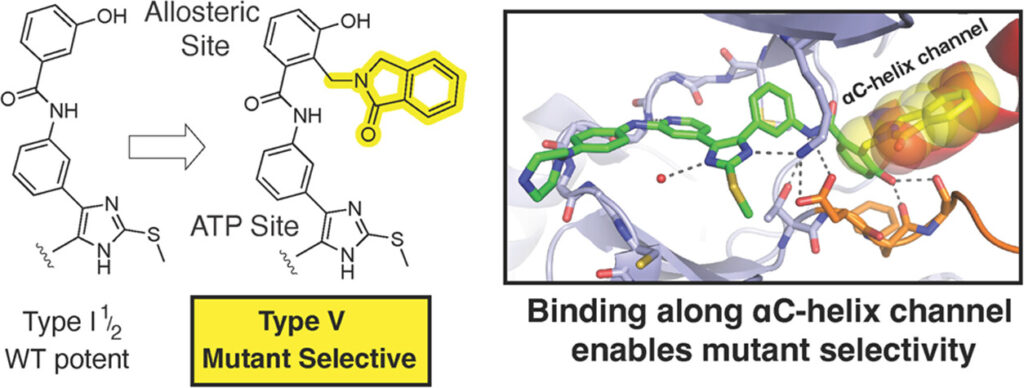
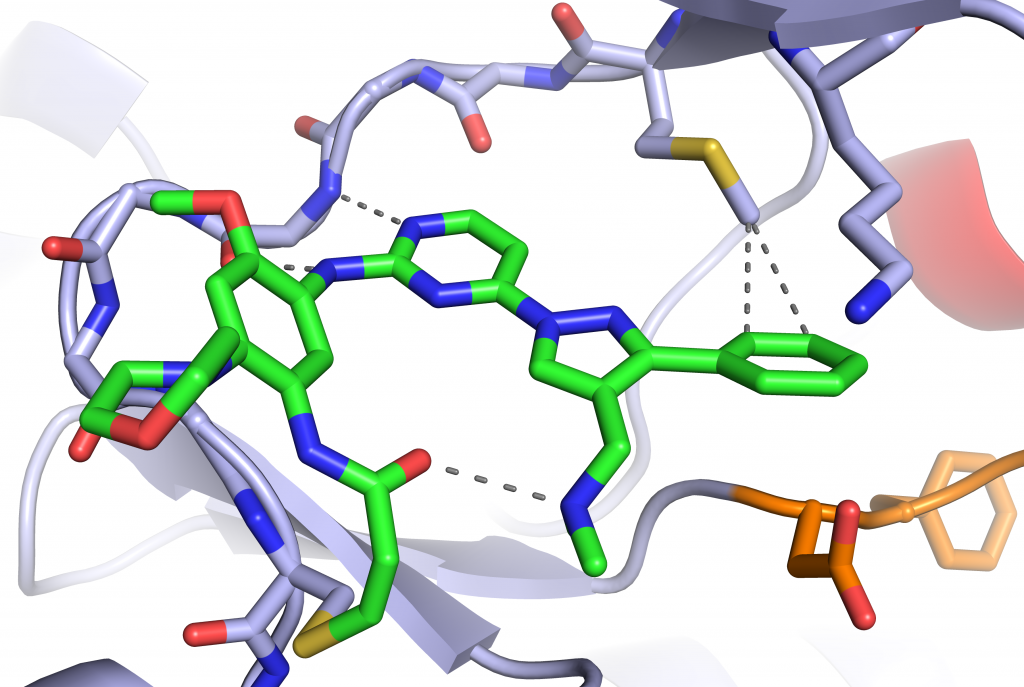
- Structural Studies of Fourth-Generation EGFR Inhibitors. ACS Medicinal Chemistry Letters 2026.
- Lazertinib: breaking the mold of third-generation EGFR inhibitors. RSC Medicinal Chemistry 2025.
- Tilting the Scales toward EGFR Mutant Selectivity: Expanding the Scope of Bivalent “Type V” Kinase Inhibitors. Journal of Medicinal Chemistry 2024.
- Structural Basis for Inhibition of Mutant EGFR with Lazertinib (YH25448). ACS Medicinal Chemistry Letters 2022.