Pre-print Manuscripts During Independent Career at UB († Corresponding Author)
Publications During Independent Career at UB († Corresponding Author)
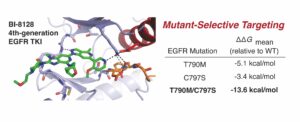
(30) Tahereh Damghani, Shenghan Song, Kaly S. Lin, Jianing Li, David E. Heppner† “Structural Studies of Fourth-Generation EGFR Inhibitors Reveal Insights into Selective T790M and C797S targeting.” ACS Medicinal Chemistry Letters 2026 https://doi.org/10.1021/acsmedchemlett.5c00725
(29) David E. Heppner,† Simone V. Bigi-Botterill, Andrew P. Riley, Kelly Chibale, Craig W. Lindsley “Structural Biology Enabling Drug Discovery.” Journal of Medicinal Chemistry 2025. https://doi.org/10.1021/acs.jmedchem.5c03093
(28) Meizhong Jin, Hayley Binch, David E. Heppner, Philip Jones “Introduction to the themed collection on kinases.” RSC Medicinal Chemistry 2025 https://doi.org/10.1039/D5MD90032H
(27) David E. Heppner† “Proposed Cuts to Health Research Threaten the Future of Drug Discovery and Development” Journal of Medicinal Chemistry 2025 https://doi.org/10.1021/acs.jmedchem.5c02080
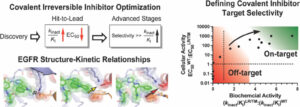
(26) Tahereh Damghani, Surbhi P. Chitnis, Omobolanle A. Abidakun, Kishan B. Patel, Kaly S. Lin, Emily A. Ouellette, Abigail M. Lantry, David E. Heppner†“Profiling and Optimizing Targeted Covalent Inhibitors through EGFR-Guided Studies.” Journal of Medicinal Chemistry 2025 https://doi.org/10.1021/acs.jmedchem.5c01661
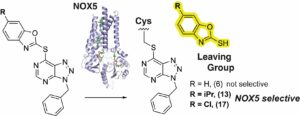
(25) Blessing C. Ogboo, Kishan B. Patel, Marta Massari, Sara Machese, Joana Reis, Emily Joyce, Miao-chong J. Lin, Johnathan Rabb, Omobolanle Abidakun, Qing Lin, Albert van der Vliet, Andrea Mattevi, David E. Heppner† “Enhancing selectivity and potency of SNAr Covalent Inhibitors of NADPH Oxidase Enzymes.” Journal of Medicinal Chemistry 2025 https://doi.org/10.1021/acs.jmedchem.5c01272
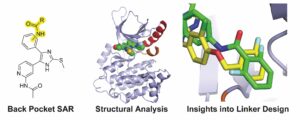
(24) Surbhi P. Chitnis, Florian Wittlinger, Mareike Möllers, Tyler J. Hartman, Marcel Günther, Michael J. Eck, Stefan A. Laufer,† David E. Heppner† “Structure-activity relationships of inactive-conformation binding EGFR inhibitors: Linking the ATP and allosteric pockets.” Archiv der Pharmazie 2025 https://doi.org/10.1002/ardp.70027
(23) David E. Heppner† “Drug Discovery and Development Faces an Ominous Future from NIH Indirect Cost Rate Cut.” Journal of Medicinal Chemistry 2025 https://doi.org/10.1021/acs.jmedchem.5c00444

(22) David E. Heppner† “Ascertaining a Structural Basis in Drug Discovery and Development” Journal of Medicinal Chemistry 2025 https://doi.org/10.1021/acs.jmedchem.5c00326
(21) David E. Heppner† “Cheat Sheet for Covalent Enzyme Inhibitors” Drug Hunter (Link) Published Jan 7, 2025

(20) Kishan B. Patel and David E. Heppner† “Lazertinib: Breaking the mold of third-generation EGFR inhibitors.” RSC Medicinal Chemistry 2025 https://doi.org/10.1039/D4MD00800F
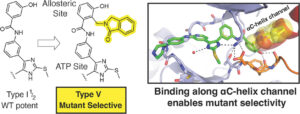
(19) Florian Wittlinger, Surbhi P. Chitnis, Calvin D. Pham, Tahereh Damghani, Kishan B. Patel, Mareike Möllers, Ilse K. Schaeffner, Omobolanle A. Abidakun, Matthew Q. Deng, Blessing C. Ogboo, Alexander Rasch, Tyler S. Beyett, Brian Buckley, Frederic Feru, Tatiana Shaurova, Cornelius Knappe, Michael J. Eck, Pamela A. Hershberger, David A. Scott, Asher L. Brandt, Stefan A. Laufer†, and David E. Heppner† “Tilting the Scales toward EGFR Mutant Selectivity: Expanding the Scope of ‘Type V’ Kinase Inhibitors” Journal of Medicinal Chemistry 2024 https://doi.org/10.1021/acs.jmedchem.4c02311
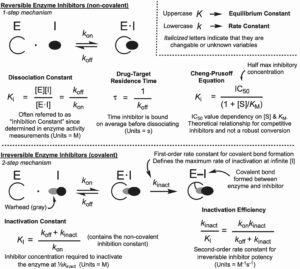
(18) David E. Heppner,† Blessing C. Ogboo, Daniel A. Urul, Earl W. May, Erik W. Schaefer, Andrew S. Murkin, and Matthias Gehringer. “Demystifying Functional Parameters for Irreversible Enzyme Inhibitors.” Journal of Medicinal Chemistry 2024 https://doi.org/10.1021/acs.jmedchem.4c01721
(16 & 17) David E. Heppner, Simone V. Bigi-Botterill, Andrew P. Riley, Kelly Chibale, and Craig W. Lindsley. “Structural Biology in Drug Discovery and Development: Call for Papers.” Journal of Medicinal Chemistry & ACS Medicinal Chemistry Letters 2024 https://doi.org/10.1021/acs.jmedchem.4c01492 https://doi.org/10.1021/acsmedchemlett.4c00318

(15) Tyler S. Beyett, Jaimin K. Rana, Ilse K. Schaeffner, David E. Heppner, and Michael J. Eck “Structural analysis of the macrocyclic inhibitor BI-4020 binding to EGFR kinase.” ChemMedChem 2024 https://doi.org/10.1002/cmdc.202300343
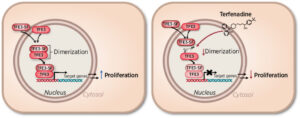
(14) Nur P. Damayanti, Ricardo A. Cordova, Christopher Rupert, Ilaria Delle Fontane, Li Shen, Sabrina Orsi, Angela J. Klunk, W. Marston Linehan, Kirk A. Staschke, Peter C. Hollenhorst, David E. Heppner, Roberto Pili. “TFE3-splicing factor fusions represent functional drivers and draggable targets in translational renal cell carcinoma.” Cancer Research 2024 https://doi.org/10.1158/0008-5472.CAN-23-1789
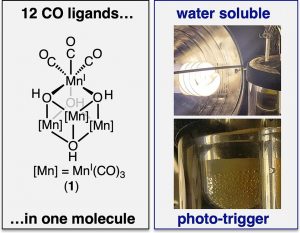
(13) David C. Lacy,† David E. Heppner,† Brian Buckley, Justin Griffiths, Parami S. Gunasekera, Jully Patel, Zuleydian Roche-Rivera, Kelsey Coppola, Kyle Dempsey, Shweta C. Pillai, Bryan R. Renzoni Twelve CO molecules for the price of one. A simple water-soluble organometallic CORM, [Mn(CO)3(µ3-OH)]4. Polyhedron 2024 251 116859. https://doi.org/10.1016/j.poly.2024.116859
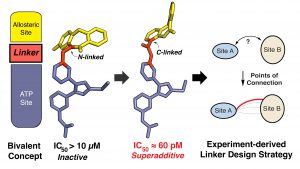
(12) Florian Wittlinger,# Blessing C. Ogboo,# Ekaterina Shevchenko, Tahereh Damghani, Calvin D. Pham, Ilse K. Schaeffner, Brandon T. Oligny, Surbhi P. Chitnis, Tyler S. Beyett, Alexander Rasch, Brian Buckley, Daniel A. Urul, Tatiana Shaurova, Earl W. May, Erik M. Schaefer, Michael J. Eck, Pamela A. Hershberger, Antti Poso, Stefan A. Laufer†, David E. Heppner† “Linking ATP and allosteric sites to achieve superadditive binding with bivalent EGFR kinase inhibitors.” Communications Chemistry 2024, 7, Article number 38. https://www.nature.com/articles/s42004-024-01108-3
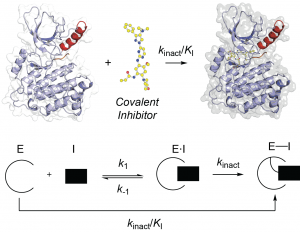
(11) Kristopher W. Hoyt, Daniel A. Urul, Blessing C. Ogboo, Florian Wittlinger, Stefan A. Laufer, Erik M. Schaefner, † Earl W. May,† and David E. Heppner.† “Pitfalls and considerations in determining the potency and mutant selectivity of covalent epidermal growth factor receptor inhibitors.” Journal of Medicinal Chemistry 2024, 64, 2-16. doi.org/10.1021/acs.jmedchem.3c01502
-
- Highlighted in Practical Fragments (Dan Erlanson)
(10) Justin Kim, Ilse Schaeffner, David E. Heppner, Ciric To, Pasi Jänne, Tyler S. Beyett and Michael J. Eck “A Constitutive EGFR Kinase Dimer to Study Inhibitor Pharmacology.” Molecular Pharmacology 2024 105 97-103. doi.org/10.1124/molpharm.123.000768
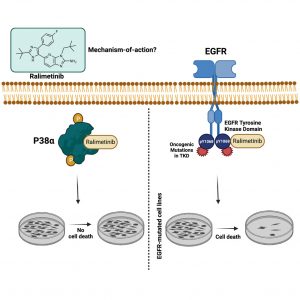
(9) Debanjan Bhattacharjee, Jaweria Bakar, Surbhi P. Chitnis, Erin L. Sausville, Kumar Dilip Ashtekar, Brianna E. Mendelson, Kaitlin Long, Joan C. Smith, David E. Heppner,† and Jason M. Sheltzer†. “Inhibition of a lower-potency target drives the anti-cancer activity of a clinical p38 inhibitor.” Cell Chemical Biology 2023, 30, 1211-1222 10.1016/j.chembiol.2023.09.013
(8) David E. Heppner† “Design and development of mutant EGFR inhibitors from a structural perspective.” Indian Journal of Biochemistry and Biophysics 2023, 60, 645-650. https://doi.org/10.56042/ijbb.v60i9.3967 Invited Review based on talk given at the Celebrating Proteins Conference in Vadodara, India.
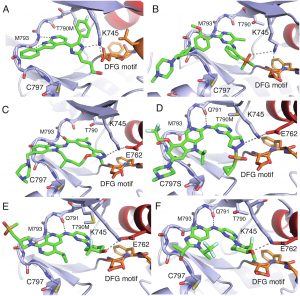
(7) Tahereh Damghani, Florian Wittlinger, Tyler S. Beyett, Michael J. Eck, Stefan A. Laufer, David E. Heppner† “Structural elements that enable specificity for mutant EGFR kinase domains with next-generation small-molecule inhibitors.” Methods in Enzymology Invited Contribution. 2023 https://doi.org/10.1016/bs.mie.2023.03.013
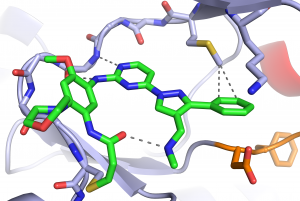
(6) David E. Heppner,† Florian Wittlinger, Tyler S. Beyett, Tatiana Shaurova, Daniel A. Urul, Brian Buckley, Calvin D. Pham, Ilse Schaeffner, Bo Yang, Blessing Ogboo, Earl W. May, Erik M. Schaefer, Michael J. Eck, Stefan A. Laufer, Pamela A. Hershberger. “Structural Basis for Inhibition of Mutant EGFR with Lazertinib (YH25448)” ACS Med. Chem. Letts. 2022 https://pubs.acs.org/doi/full/10.1021/acsmedchemlett.2c00213
(5) Yoshihisa Kobayashi, Geoffrey R. Oxnard, Elizabeth F. Cohen, Navin R. Magadevan, Joao V. Alessi, Yin P. Hung, Arrien A. Bertram, David E. Heppner, Mauricio F. Rieiro, Karina P. Sacardo, Rodrigo Saddi, Mariana P. Macedo, Radael B. Blasco, Jiaqi Li, Kari J. Kurppa, Tom Nguyen, Emma Voligny, Guruprasad Ananda, Roberto Chiarle, Artur Katz, Machael Y. Tolstorukov, Lynette M. Sholl, and Pasi A. Jänne. “Genomic and biological study of fusion genes as resistance mechanisms to EGFR inhibitors.” Nature Communications 2022, 13, 5614.
(4) Blessing C. Ogboo, Uriy V. Grabovyy, Aniket Maini, Scott Scouten, Albert van der Vliet, Andrea Mattevi, & David E. Heppner† “Architecture of the NADPH oxidase family of enzymes”. Redox Biology 2022, 52, 102298. [Graphical Review]
(3) Christopher M. Dustin, Aida Habibovic, Milena Hristova, Caspar Schiffers, Carolyn R. Morris, Miao-Chong Joy Lin, Robert A. Bauer, David E. Heppner, Nirav Daphtary, Minara Aliyeva, and Albert van der Vliet. “Redox-dependent activation of Src kinase mediates epithelial IL-33 production and signaling during acute airway allergen challenge.” The Journal of Immunology 2021, 12, 2989.
(2) David E. Heppner† & Michael J. Eck† “A structural perspective on targeting the RTK/Ras/MAP kinase pathway in cancer”. Protein Science 2021, 30, 1535. PMCID: PMC8284588 [Review]
(1) David E. Heppner† “Structural insights into redox-active cysteine residues of the Src family kinases”. Redox Biology 2021, 41, 101934. PMCID: PMC8022254 [Review]
Research Publications (*Equal contributions)
(25) Tyler S. Beyett, Ciric To, David E. Heppner, Jaimin K. Rana, Anna M. Schmoker, Jaebong Jang, Dries J. H. De Clercq, Gabriel Gomez, David A. Scott, Nathanael S. Gray, Pasi A. Jänne, and Michael J. Eck. “Molecular basis for cooperative binding and synergy of ATP-site and allosteric EGFR inhibitors.” Nature Communications 2022, 13, 2530.
(24) Thomas W. Gero, David E. Heppner, Tyler S. Beyett, Ciric To, Seth C. Azevedo, Jaebong Jang, Thomas Bunnell, Frederic Feru, Zhengnian Li, Bo Hee Shin, Kara M Soroko, Prafulla Gokhale, Nathanael S. Gray, Pasi A. Jänne, Michael J. Eck, and David A. Scott. “Quinazolinones as allosteric fourth-generation EGFR inhibitors for the treatment of NSCLC.” Bioorganic and Medicinal Chemistry Letters 2022, 3, 402-217.
(23) Ciric To, Tyler S. Beyett, Jaebong Jang, William W. Feng, Magda Bahcall, Heidi M. Haikala, Bo H Shin, David E. Heppner, Jaimin K. Rana, Brittaney A. Leeper, Kara M. Soroko, Michael J Poitras, Prafulla C. Gokhale, Yoshihisa Kobayashi, Kamal Wahid, Kari J. Kurppa, Thomas W. Gero, Michael Cameron, Atsuko Ogino, Mierzhati Mushajing, Chunxiao Xu, Yanxi Zhan, David A. Scott, Michael J. Eck, Nathanael S. Gray, Pasi A. Jänne. “An allosteric inhibitor against the therapy-resistant mutant forms of EGFR in non-small cell lung cancer” Nature Cancer 2022, 3, 402-417.
(22) Florian Wittlinger,* David E. Heppner,* Marcel Günther, Ciric To, Bo Hee See, Jaimin K. Rana, Anna M. Schmoker, Tyler S. Beyett, Lena M. Berger, Benedict-Tilman Berger, Nicolas Bauer, James D. Vasta, Cesear R. Corona, Matthew B. Roberts, Stefan Knapp, Pasi A. Jänne, Michael J. Eck, & Stefan A. Laufer “Design of a ‘two-in-one’ mutant-selective epidermal growth factor receptor inhibitor that spans the orthosteric and allosteric sites.” J Med. Chem. 2022, 65, 1370.
(20) Stephen M. Jones, David E. Heppner, Kenneth Vu, Daniel J. Kosman, & Edward I. Solomon “Rapid Decay of the Native Intermediate in the Metallooxidase Fet3p Enables Controlled FeII Oxidation for Efficient Metabolism.” J Am. Chem. Soc. 2020, 142, 10087. PMCID: PMC67315797
(19) David E. Heppner, Marcel Günther, Florian Wittlinger, Stefan Laufer, & Michael J. Eck “Structural basis for EGFR inhibition by trisubstituted imidazole inhibitors. J. Med. Chem. 2020, 63, 4293. PMCID: PMC7730863
(18) Haopeng Xiao, Mark P. Jedrychowski, Devin K. Schweppe, Edward L. Huttlin, Qing Yu, David E. Heppner, Jiaming Li, Jiani Long, Evanna L. Mills, John Szpyt, Zhixiang He, Guangyang Du, Ryan Garrity, Anita Reddy, Laura P. Vaites, Joao A. Paolo, Tinghu Zhang, Nathanael S. Gray, Steven P. Gygi, & Edward T. Chouchani “A quantitative tissue-specific landscape of protein redox regulation during aging.” Cell 2020, 180, 968.
(17) Dries J. H. De Clercq,* David E. Heppner,* Ciric To, Jaebong Jang, Eunyoung Park, Cai-Hong Yun, Mierzhati Mushajiang, Bo Hee Shin, David A. Scott, Pasi A. Jänne, Michael J. Eck, & Nathanael S. Gray “Discovery and optimization of dibenzodiazepinones as allosteric mutant-selective EGFR inhibitors.” ACS Med. Chem. Lett. 2019, 10, 1549. PMCID: PMC6862338
(16) Ciric To, Jaebong Jang, Ting Chen, Eunyoung Park, Mierzhati Mushajiang, Dries J. H. De Clercq, Man Xu, Stephen Wang, Michael D. Cameron, David E. Heppner, Bo Hee Shin, Thomas W. Gero, Annan Yang, Suzanne E. Dahlberg, Kwok-Kin Wong, Michael J. Eck, Nathanael S. Gray, & Pasi A. Jänne. “Single and dual targeting of mutant EGFR with an allosteric inhibitor.” Cancer Discovery 2019, 9, 926. PMCID: PMC6664433
(15) Andrew C. Little, Milena Hristova, Loes van Lith, Caspar Schiffers, Christopher M. Dustin, Aida Habibovic, Karamatullah Danyal, David E. Heppner, Maio-Chong J. Lin, Jos van der Velden, Yvonne M. Janssen-Heininger, & Albert van der Vliet. “Dysregulated redox regulation contributes to nuclear EGFR localization and pathogenicity in lung cancer.” Scientific Reports 2019, 9, 4844. PMCID: PMC63425021
(14) Nicolas Chamberlain, Bethany R. Mihavics, Emily M. Nakada, Sierra R. Bruno, David E. Heppner, David G. Chapman, Sidra M. Hoffman, Albert van der Vliet, Benjamin T. Suratt, Oliver Dienz, John F. Alcorn & Vikas Anathy “Lung epithelial protein disulfide isomerase A3 (PDIA3) plays an important role in influenza infection, inflammation, and airway mechanics.” Redox Biology 2019, 22, 101129. PMCID: PMC6365984
(13) David E. Heppner,* Christopher M. Dustin,* Chenyi Liao,* Milena Hristova, Carmen Veith, Andrew C. Little, Bethany A. Ahlers, Sheryl L. White, Bin Deng, Ying-Wai Lam, Jianing Li, & Albert van der Vliet “Direct cysteine sulfenylation drives activation of the Src kinase.” Nature Communications 2018 9, 4522. PMCID: PMC6207713
(12) David E. Heppner, Milena Hristova, Tomoaki Ida, Ana Mijuskovic, Christopher M. Dustin, Virág Bogdándi, Jon M. Fukuto, Tobias P. Dick, Péter Nagy, Jianing Li, Takaaki Akaike, & Albert van der Vliet “Cysteine perthiosulfenic acid (Cys-SSOH): A novel intermediate in thiol-based redox signaling?” Redox Biology 2018, 14, 379. PMCID: PMC5647513
(11) David E. Heppner, Yvonne M.W. Janssen-Heininger, & Albert van der Vliet “The role of sulfenic acids in cellular redox signaling: Reconciling chemical kinetics and molecular detection strategies.” Arch. Biochem. Biophys. 2017, 616, 40. PMCID: PMC5305417
(10) Aida Habibovic, Milena Hristova, David E. Heppner, Karamatullah Danyal, Jennifer L. Ather, Yvonne M.W. Janssen-Heininger, Charles G. Irvin, Matthew E. Poynter, Lennart K.A. Lundblad, Anne E. Dixon, Miklós Geiszt, & Albert van der Vliet “DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma.” JCI Insight 2016, 18, e88811. PMCID: PMC5085603
(9) David E. Heppner, Milena Hristova, Christopher M. Dustin, Karamatullah Danyal, Aida Habibovic, & Albert van der Vliet “The NADPH oxidases DUOX1 and NOX2 play distinct roles in redox regulation of epidermal growth factor receptor signaling. J Biol. Chem. 2016, 291, 23282. PMCID: PMC5087744
(8) Karamatullah Danyal, Willem de Jong, Edmund O’Brien, Robert A. Bauer, David E. Heppner, Andrew Little, Milena Hristova, Aida Habibovic, & Albert van der Vliet “Acrolein and other thiol-reactive electrophiles suppress allergen-induced innate airway epithelial responses by inhibition of DUOX1 and EGFR.” Am. J. Phys. Lung Cell. Mol. Phys. 2016, 311, L913. PMCID: PMC5130541
(7) Andrew C. Little, Derek Sham, Milena Hristova, Karamatullah Danyal, David E. Heppner, Robert A. Bauer, Lynn M. Sipsey, Aida Habibovic, & Albert van der Vliet “DUOX1 silencing in lung cancer promotes epithelial-to-mesenchymal transition, acquisition of cancer stem cell characteristics, and invasive properties.” Oncogenesis 2016, 5, e261. PMCID: PMC5117847
(6) David E. Heppner, Christian H. Kjaergaard, & Edward I. Solomon “Mechanism of the reduction of the native intermediate in the multicopper oxidases: Insights into rapid intramolecular electron transfer in turnover.” J. Am. Chem. Soc. 2014, 136, 17788. PMCID: PMC4291763
(5) David E. Heppner, Christian H. Kjaergaard, & Edward I. Solomon “Molecular origin of rapid versus slow intramolecular electron transfer in the catalytic mechanism of the multicopper oxidases.” J. Am. Chem. Soc. 2013, 135, 12212. PMCID: PMC3807568
(4) Bryan T. Op’t Holt, Michael A. Vance, Liviu M. Mirica, David E. Heppner, T. Daniel P. Stack, & Edward I. Solomon “Reaction coordinate of a functional model of tyrosinase: Spectroscopic and computational characterization.” J. Am. Chem. Soc. 2009, 131, 6421. PMCID: PMC2692929
(3) John L. Lewin, David E. Heppner, & Christopher J. Cramer “Validation of density functional modeling protocols on experimental bis(µ-oxo)/µ-η2:η2-peroxo dicopper equilibria.”J. Biol. Inorg. Chem. 2007, 12, 1221. PMID: 17710449
(2) David E. Heppner, Benjamin F. Gherman, William B. Tolman, & Christopher J. Cramer “Can an ancillary ligand lead to a thermodynamically stable end-on 1:1 Cu-O2 adduct supported by the β-diketiminate ligand?” Dalton Trans. 2006, 40, 4773. PMID: 17033702
(1) Benjamin F. Gherman, David E. Heppner, William B. Tolman, & Christopher J. Cramer “Models for dioxygen activation by the CuBsite of dopamine β-monooxygenase and peptidylglycine α-hydroxylating monooxygenase. J Biol. Inorg. Chem. 2006, 11, 197-205. PMID: 16344970
Reviews, Chapters, and Commentaries
(9) Albert van der Vliet, Christopher M. Dustin, & David E. Heppner “Redox regulation of protein kinase signaling.” Chapter 16 in “Oxidative Stress: Eustress and Distress,” H. Sies Ed., Elsevier, 2020. [Book Chapter]
(8) Christopher M. Dustin, David E. Heppner, Maio-Chong J. Lin & Albert van der Vliet “Redox regulation of tyrosine kinase signaling: More than meets the eye” J. Biochem. 2020,167, 151. PMCID: PMC6988750 [Review]
(7) David E. Heppner, Tyler S. Beyett, & Michael J. Eck “A driving test for oncogenic mutations.” J. Biol. Chem. 2019, 24, 9390. PMCID: PMC6576455 [Editors’ Pick Highlight]
(6) Albert van der Vliet, Karamattulah Danyal, & David E. Heppner “Dual oxidase: A novel therapeutic target in allergic disease.” Br. J. Pharmacol. 2018, 175, 1401. PMCID: PMC5900994 [Review]
(5) Andrew C. Little, Arvis Sulovari, Karamatullah Danyal, David E. Heppner, David J. Seward, & Albert van der Vliet “Paradoxical roles of dual oxidases in cancer biology.” Free Radic. Biol. Med. 2017, 110, 117. PMCID: PMC5535817 [Review]
(4) David E. Heppner & Albert van der Vliet “Redox-dependent regulation of epidermal growth factor receptor signaling.” Redox Biology 2016, 8, 24. PMCID: PMC4710793 [Minireview]
(3) Edward I. Solomon, Christian H. Kjaergaard, & David E. Heppner “Molecular properties and reaction mechanism of multicopper oxidases related to their use in biofuel-cells.” In “Electrochemical Processes in Biological Systems,” A. Lewenstam & L. Groton Eds., John Wiley & Sons, Inc., Hoboken, N.J. 2015. [Book Chapter]
(2) Edward I. Solomon, David E. Heppner, Esther M. Pierce, Jake W. Ginsbach, Jordi Cirera, Munzarin Qayyum, Matthew T. Kieber-Emmons, Christian H. Kjaergaard, Ryan G. Hadt, & Li Tian “Copper active sites in biology.” Chem. Rev. 2014, 114, 3659. PMCID: PMC4040215 [Review]
(1) Edward I. Solomon, Jake W. Ginsbach, David E. Heppner, Matthew T. Kieber-Emmons, Christian H. Kjaergaard, Pieter J. Smeets, Li Tian, & Julia S. Woertink. “Copper dioxygen (bio)inorganic chemistry.” Faraday Discussions 2011, 148, 11. PMCID: PMC3062954 [Review]
